100 Trillion Reasons You’re Fat, Sick and Depressed (Part 1)
Is it possible that the 100 trillion reasons you might be fat, sick and depressed are due to single-cell bacteria that proliferate your gut (among other places)? Prepare to be amazed.
Photo credit: Mother Jones
This article is adapted from "BioHack #6" in the 12 Ageproof BioHacks course. When it's finished, I'll send it to you FREE. Please put your email address in that blue box to the right.
If you sometimes feel fat, sick and depressed, a trillion tiny things might be the reason.
Your human body is composed of about 10 trillion cells. That’s a mighty big number, but there’s more to you than you think. In fact, ten times more.
I’m referring to your microbiome, the community of thousands of species of microbes – bacteria — that has colonized nearly every part of you from your nose down through your stomach and intestines to your anus.
If you put bacteria illuminating goggles on and looked at yourself in the mirror, you’d see something like this:
There are approximately 100 trillion single-cell bacteria in you, ten times more than the human cells that make you. And they’re not just hanging out passively. They are hungry. They have influence. They are essential to good health.
Ignore them at your peril.
Scientists are discovering that these single-cell bacteria have a profound affect on your health in nearly every way imaginable. Among a long list, they:
- Help to extract energy from the food you eat,
- Affect your immune system (for better or worse),
- Promote or reduce inflammation, and
- Influence your body composition – thin or fat.
In this article, I’ll cover:
- How in the heck are there more bacteria than us in us; and
- How this bacteria — microbiota — affect our body composition, immune system and brain — basically making us fat, sick and depressed. Or… just the opposite.
To get you warmed up, check out this video reported Moises Velasquez-Manoff on MotherJones.com:
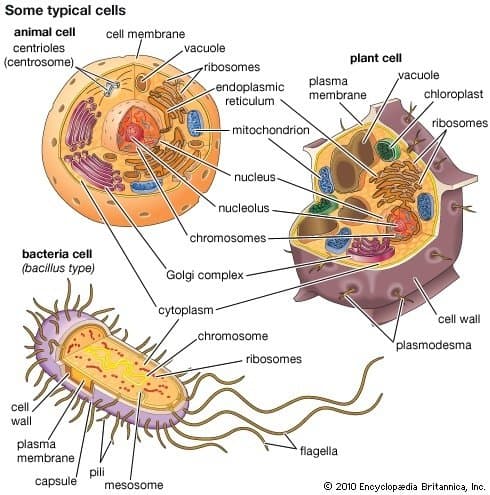
Human Cell vs Bacterium Cell
The human cells in your body are fairly complicated biochemical machines. Each one has a nucleus, cell membrane and a complex energy production “factory” housed in the mitochondria.
Bacteria are single-celled organisms that lack a membrane-bound nucleus and other internal structures. They are perhaps 1/100th the size of a human cell, about one micrometer long. Bacteria are completely independent organisms able to eat and reproduce – they are sort of like fish swimming in the rivers of your body.
Under the right conditions bacteria reproduce very quickly: One bacterium divides into two separate bacteria perhaps once every 20 or 30 minutes. At that rate, one bacterium can become millions in just a few hours!
They’re smaller, yes, but because the bacteria in us are so numerous, have their own biochemical imperatives and develop their own ecosystems, they are a potent force that must be our ally, not our foe.
In a hallmark study in 2011, researchers at the University of Maryland School of Medicine identified 26 species of bacteria in the human gut microbiota that appear to be linked to obesity and related metabolic complications. These include:
- Insulin resistance,
- High blood sugar levels,
- Increased blood pressure, and
- High cholesterol.
Collectively known as “metabolic syndrome,” these biomarkers significantly increase an individual’s risk of developing diabetes, cardiovascular disease and stroke.
This is a very significant discovery!
Is it possible to reduce or extinguish a major precursor to the most pervasive chronic diseases afflicting people worldwide simply be adjusting the bacterial in our gastrointestinal pathway?
Claire M. Fraser, Ph.D., professor of medicine and microbiology and immunology and director of the Institute for Genome Sciences (IGS) at the University of Maryland School of Medicine, said:
We identified 26 species of bacteria that were correlated with obesity and metabolic syndrome traits such as body mass index (BMI), triglycerides, cholesterol, glucose levels and C-reactive protein, a marker for inflammation. We can’t infer cause and effect, but it’s an important step forward that we’re starting to identify bacteria that are correlated with clinical parameters, which suggests that the gut microbiota could one day be targeted with medication, diet or lifestyle changes.
OK, Dr. Claire Fraser, we’re going to do just that — look into diet and lifestyle changes (mostly in Part 2), but first let’s dig into how the bacteria in us influences our body composition, immune system and brains, making many of us fat, sick and depressed.
Belly Fat Effect and Your Microbes
While it’s true that overeating and the lack of exercise are the primary causes for being overweight (irrespective of where you carry the poundage on your body), there are other significant factors, such as the type and population of bacteria in your gut.
Obese people have less gut flora (bacteria) than lean people, and this may have to do with two types of bacteria: Firmicutes and Bacteroidetes.
Firmicutes are dominant in fat people and Bacteroidetes are dominant in thin people. Researchers say that when there’s an increase in Firmicutes and a corresponding decrease in Bacteroidetes, fat happens. (Source.)
Much of the research about the microbiome is, unsurprisingly, done on animals; namely mice and rats. Mice that receive gut bacteria transplants from obese people gain more weight and fat mass than mice getting bacteria from lean people. Moreover, the transplants change mice metabolism, and – most likely – ours as well.
Animals that receive gut bacteria from an obese person have metabolic changes linked with obesity in humans (such as increased production of compounds called branched-chain amino acids); while those that received gut bacteria from a lean person had metabolic changes linked with reduced body weight (such as increased breakdown of carbohydrates).
The available evidence suggests that about two-thirds of the insulin response to an oral glucose load (eating grain-based carbohydrates, for instance) is due to the potentiating effect of gut-derived incretin hormones; therefore, if incretin signaling is diminished, it could result in insufficient insulin being produced by the pancreas to shuttle the glucose load into cells for energy metabolism. (Source)
To be clear about this: If you have either insufficient insulin production or you are insulin resistant, irrespective of the amount being produced by your pancreas, you can not adequately turn glucose (primarily from carbs; secondarily, protein) into the fuel that runs your body. When this happens, fat happens because this excess glucose gets stored as fat.
This might explain the “Yo-yo Diet” phenomena.  Yo-yo dieting refers to diets where the dieter loses weight and then gains it back, again and again, up and down, like a yo-yo. For many, this is the only experience they have with dieting – lose some weight, gain it back, then do it again, ad infinitum.
Yo-yo dieting refers to diets where the dieter loses weight and then gains it back, again and again, up and down, like a yo-yo. For many, this is the only experience they have with dieting – lose some weight, gain it back, then do it again, ad infinitum.
This may have something to do with the just-mentioned incretin hormones.
This is important, so let’s review it again…
Incretins are gut hormones that are secreted from enteroendocrine cells into the blood within minutes after eating. One of their many physiological roles is to regulate the amount of insulin that is secreted after eating.
Insulin is needed to transport the blood glucose resulting from eating carbohydrates and (to a lesser extent) protein into cells for energy metabolism. Low calorie diets are associated with a rapid reduction of incretin signaling, leading to weight regain.
This has been studied in various low calorie-dieting populations. Incretin levels were measured before dieting, during the diet and up to one year after the diet. The low calorie diets suppressed incretin during the diet, and it remained that way for a year later, thus If the glucose is not metabolized, it gets stored as fat.
There’s one more thing. Something that seems preposterous.
When it comes to food selection, our bacteria are puppet masters, and we’re the puppets.
Dr. Carlo Maley of the University of California San Francisco touches on this when he says:
“Bacteria within the gut are manipulative. There is a diversity of interests represented in the microbiome, some aligned with our own dietary goals and others not.” (Source)
The question is whether these bacteria can influence what we eat to their benefit. Many microbiome researchers believe they can.
The likely process: The bacteria release molecules into our digestive system that are transmitted through the immune, endocrine and nervous systems to signal the brain what to put in our mouths.
“Think of it as the bacteria’s way of ordering fast food”, says Steven Luntz, author of Are Your Bacteria Making You Fat.
The theory that microbes have a hand in puppet mastery has been tested in mice (of course).
The test: Engineer mice to have excessive appetites — which led to insulin resistance, high blood pressure, elevated levels of cholesterol and triglycerides, fatty liver disease, and a 20% increase in body weight — and then transplant their microbiota into normal mice.
What do you think happened to the “normal” mice?
They developed the very same metabolic syndrome abnormalities that existed in the engineered mice! The overgrowth of bacteria in the microbiota of the engineered mice fueled obesity and triggered unhealthy food cravings in the normal mice. (Source)
Do you want want to lose body fat, and think you’ve tried everything? Well, maybe you need to check your microbiota, and begin consuming the “4 Ps”. But I’m getting ahead of myself, because we have yet to investigate our bacteria’s effect on the immune system and brain — not to mention that the “Ps” will be covered in Part 2, not here.
Your Immune System Is In Your Gut
Photo credit: npr.org/blogs/goatsandsoda/2014/11/14/363375355/how-bacteria-in-the-gut-help-fight-off-viruses”
Here’s a surprise:
“The human gastrointestinal tract… constitutes about seventy percent of the immune system.”(Source)
This relationship begins at birth.
In a 2012 study, researchers Robert Chapkin of Texas A&M University, and Iddo Friedberg of Miami University in Ohio learned that babies fed breast milk had more diversity in their gut microflora (their microbiome) than did babies fed formula, concluding that both food sources influence the composition of the babies’ gut bacteria. Also discovered was a link between genes “turned on” in the babies’ gut bacteria and the genes activated in their immune systems. (Source)
Gut bacteria are instrumental in the development of innate immune cells (macrophages, monocytes and neutrophils), which are special white blood cells that provide a first line of defense against invading pathogens. These white blood cells circulate in the blood and are stored both in the spleen and in bone marrow.
Given that these specialized white blood cells are critical contributors to an effective immune system, how may they be affected by microbiota?
New research from the RIKEN Center for Integrative Medical Sciences in Japan tries to answer that question. Their aim was to illuminate the role of gut bacteria on the immune system.
When the Japanese team compared counts of white blood cells in mice born without gut bacteria (“good” or “bad”) – known as “germ-free” mice – and healthy mice with a normal gut bacteria population, they found the germ-free mice had fewer white blood cells.
The germ-free mice also had fewer stem-like cells that can differentiate into some types of immune cells. Plus, their spleens contained defective innate immune cells whose populations never reached the size found in healthy mice with microbes in their gut.
The researchers tested the mice’s ability to fight off infection by exposing them to the bacterium Listeria monocytogenes, which is harmful to humans, and often used in immune system studies using mice.
These were the results:
- Mice with beneficial bacteria recovered quickly after being injected with Listeria monocytogenes.
- Mice that were “germ-free mice”, having no beneficial bacteria, died because their immune systems could not fight off the infection, but when that mouse population were given gut bacteria typical of healthy mice, their white cell count increased, and they survived the infection.
- Healthy mice given antiobiotics to kill their gut bacteria, and then injected with Listeria got sick and had trouble fighting off the infection.
Antibiotics kill the gut bacteria that support immunity against infections. This has wide-ranging implications for humans. When patients are given antibiotics for surgeries or the flu, it’s likely that their gut microbe population is damaged, thereby making them more susceptible to infections.
The potential for microbial populations to affect the immune system was studied by the RIKEN team at the molecular level. They investigated the molecular mechanisms by which commensal microbes increase the number of regulatory T cells (Treg cells) present in the colon of mice that were bred germ-free.
(“Commensal microbes” are beneficial bacteria that provide the host — in this case, mice — with essential nutrients. “Treg cells” are a subpopulation of T cells that modulate the immune system and suppress autoimmune disease. “T cells” are a type of white blood cell that matures in the thymus.)
Their research demonstrates that butyric acid, a short-chain fatty acid produced by commensal bacteria, acts on naïve T cells to promote their differentiation into Treg cells. It achieves this through epigenetic changes that regulate the expression of the genes responsible for differentiation of naïve T cells into Treg cells.
There are at least two very significant results from this study that could have far-reaching implications for people suffering from several chronic conditions, particularly those stemming from autoimmune disorders:
- Beneficial bacteria in the gut improves the effectiveness white blood cell immune function; and
- The butyric acid by product of the beneficial bacteria can actually transform one type of T cell (naïve T) into another type (Treg cells) that are better able to suppress autoimmune disease.
The RIKEN team leader, Dr. Hiroshi Ohno, said: “Regulatory T cells are important for the containment of excessive inflammatory responses as well as autoimmune disorders. Therefore these findings could be applicable for the prevention and treatment of inflammatory bowel disease (IBD), allergy and autoimmune disease.”
He added: “Butyrate is natural and safe as a therapy and in addition to that it is cheap, which could reduce costs for both patients and society.” (Source)
So, now we know that our microbiota can influence our body composition and immunity to disease. What, pray tell, does it do to our brains?
Brain and Behavior — Are Your Thoughts Yours?
Illustration by Benjamin Arthur for NPR
Do you think that your thoughts are your own? Think again. Gut bacteria may have an effect on the brain, and in turn, one’s behavior, studies with mice suggest.
Yes, we’re back to mice again.
Assuming you’re reading this, you are not a mouse, but scientist often study mice because how they react to experiments can be relevant to humans, not to mention that it’s easier to wire electrodes into a mouse brain and have it run through a maze than it would be with a human.
So, we’ll start with looking at some mouse studies, but also look at a landmark UCLA study on humans.
A 2011 study with mice found that disrupting their normal microbiome led to changes in their behavior and brain chemistry, making them more timid or adventurous – depending on the dominant strains of bacteria introduced in their gastrointestinal tract.
At the time, it wasn’t clear that the same thing happens in humans, but the findings did explain why some gastrointestinal diseases, such as irritable bowel syndrome, are often associated with disorders that can affect behavior, including depression and anxiety.
To investigate the link between bacteria in the microbiome and behavior, researcher Stephen Collins and his colleagues gave healthy mice antibiotics which they knew would disturb and compromise their natural gut bacteria. The mice became less anxious. When their gut bacteria was restored to normal, so was their behavior. Control mice that were given water instead of antibiotics showed no changes in behavior. Mice that didn’t have any gut bacteria also showed no changes in behavior when they received antibiotics. (Source)
As mentioned, disrupting the contents of the gut also appears to affect brain chemistry. Mice given antibiotics had an increased amount of a brain protein called derived neurotrophic factor, (“BDNF”) in their brains compared to control mice. Changes in the levels of BDNF have been previously linked to depression and anxiety.
The researchers also did some gut bacteria swapping. Different strains of mice are known to exhibit different behavior patterns — some are more anxious while others are aggressive and hyperactive. The researchers took mice from both extremes and exchanged their gut bacteria. They saw the behavior flipped as well — the anxious mice became more active and daring and the aggressive mice became more passive.
Given these results, unsurprisingly, the researchers suspect the bacteria are producing chemicals that can access and influence the brain.
And not only in mice.
UCLA researchers have evidence that bacteria ingested in food can affect brain function in humans.
In a study of healthy women, they found that those who regularly consumed the beneficial bacteria in yogurt – called “probiotics” — showed altered brain function, both while in a resting state and in response to an emotion-recognition task. (Seems the same would be true for men.)
This is truly profound! Can you imagine that simply by feeding the beneficial microbiota in our guts, we could improve brain function?
Dr. Kirsten Tillisch, an associate professor of medicine in the digestive diseases division at UCLA’s David Geffen School of Medicine can imagine it, and has tested it. She says:
Many of us have a container of yogurt in our refrigerator that we may eat for enjoyment, for calcium or because we think it might help our health in other ways. Our findings indicate that some of the contents of yogurt may actually change the way our brain responds to the environment. When we consider the implications of this work, the old sayings ‘you are what you eat’ and ‘gut feelings’ take on new meaning. (Source)
Don’t like yogurt? No worries.
Dr. Emeran Mayer, a professor of medicine, physiology and psychiatry at the David Geffen School of Medicine at UCLA, said:
There are studies showing that what we eat can alter the composition and products of the gut flora — in particular, that people with high-vegetable, fiber-based diets have a different composition of their microbiota, or gut environment, than people who eat the more typical Western diet that is high in fat and carbohydrates. Now we know that this has an effect not only on the metabolism but also affects brain function.
So, now we know, or at least strongly suspect, that if we have the right beneficial bacteria in our gastrointestinal system we can get:
- Better body composition
- Better immunity
- Better cognition
Which begs the question: “How do we get those good critters in us”?
How do we reverse being fat, sick and depressed?
Those questions will be answered in Part 2.
There are many things you can do, but it can be distilled down to what I call the “4Ps”, the subject of next week’s article.
Stay tuned to this station.
Here’s Part 2 >Last Updated on April 11, 2023 by Joe Garma